Tirzepatide + Niacinamide Medication Injection Administration Instruction
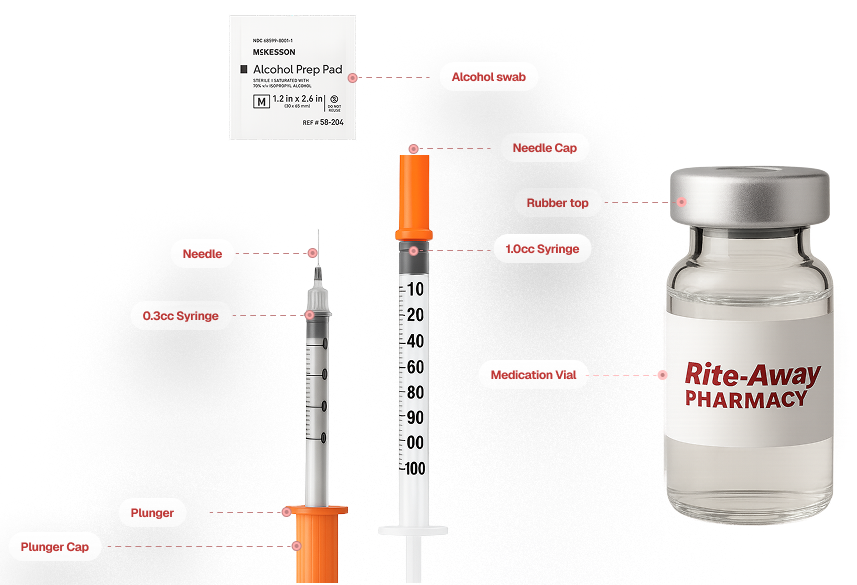

Administering The Medication
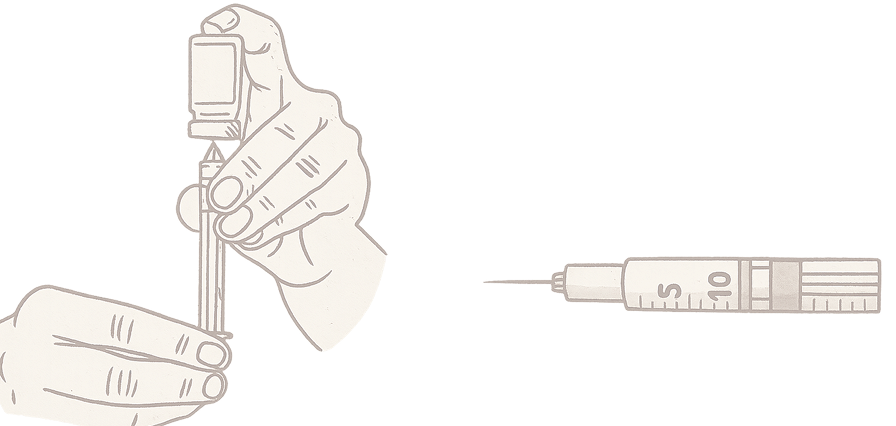
Here's how you Draw the medication and Perform the Injection
Drawing the Medication
Turn the vial upside down, insert the syringe, and pull back on the plunger to fill the syringe with your current dose.
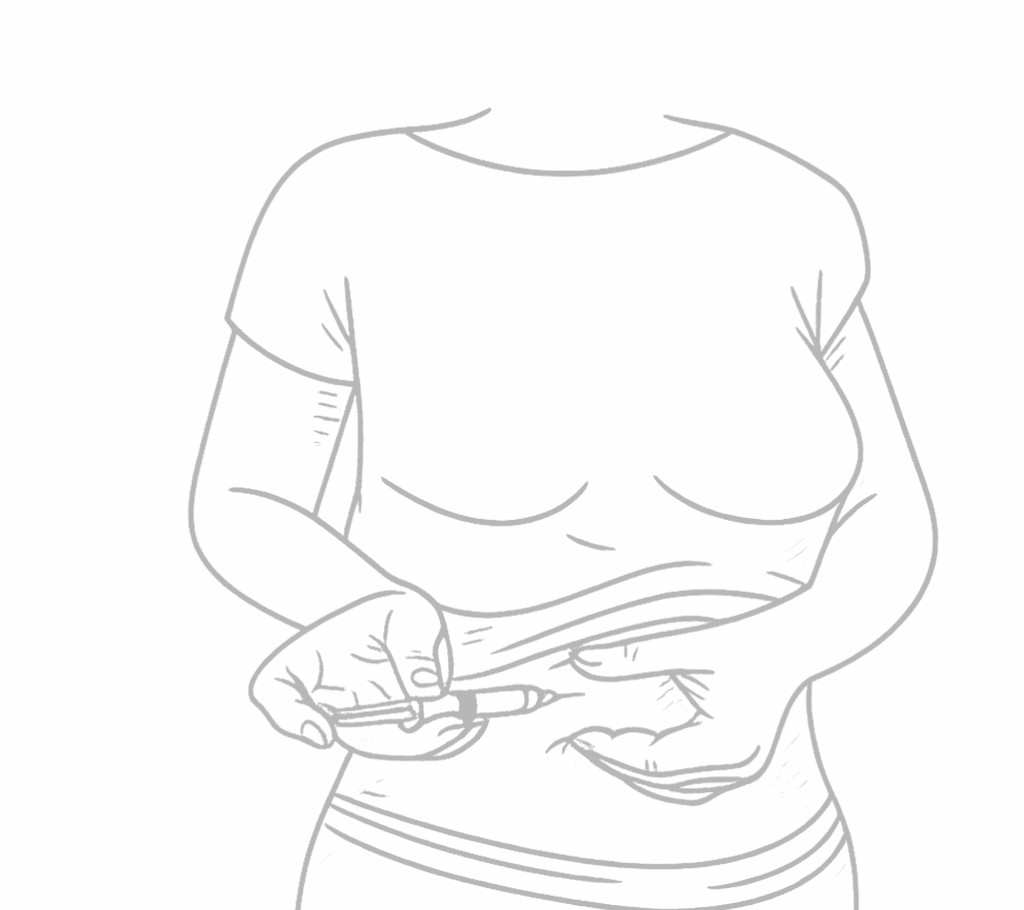
Perform the Injection
Wipe the injection site with an alcohol swab, allow it to dry.
With the other hand pinch together your belly skin between your index finger and thumb to form a small raised area. The recommended site is the fatty part of the belly.
Insert the needle into site at 45-degree angle and slowly depress the plunger, injecting the medication.
What is this compound used for?
Medication counseling is available upon request. Please ask our pharmacist if you would like more information about your prescription at 833-580-8777.


Here's everything you need to know about the Compound
How should You store the compound?
The compound should be stored in the refrigerator from 2°C to 8°C (36°F to 46°F).


Before Injecting Your Medication
Before injecting this medication, tell your healthcare provider about all of your medical conditions. Turn the vial upside down, insert the syringe, and pull back on the plunger to fill the syringe with your current dose.
Prior serious hypersensitivity reaction to tirzepatide or any of the excipients included. Symptoms include swelling of face, lips, tongue or throat, severe rash or itching, fainting or dizziness, problems breathing or swallowing, and rapid heartbeat.
You should not start or stop any medicine before you talk with the healthcare provider that prescribed this medication.
What are the possible side effects
Signs and symptoms of low sugar levels due to hypoglycemia (e.g., excessive sweating, lightheadedness, dizziness, weak or blurred vision, confusion, or increased heart rate)
Symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, dysphagia, or dyspnea)
Severe abdominal pain that radiates to the back without or with vomiting which could be pancreatitis
Hypersensitivity, rash, erythema, pruritus, dizziness, agitation, anxiety, headache, hot sensation, diaphoresis, and pain/induration at the site of SubQ injection. Pulmonary edema, congestive heart failure (CHF), peripheral vascular thrombosis, polycythemia vera (bone marrow disorder), mild transient diarrhea, itching, transitory exanthema, a feeling of swelling of the entire body has also been reported with parenteral vitamin B-containing substances.
How should I take this compound?


General Information
About the Safe and Effective Use of This Compound.
You can ask your healthcare
provider or pharmacist for information that is written for health professionals.
What are the ingredients?
Active ingredient: Tirzepatide, Niacinamide
Inactive ingredients: Benzyl Alcohol, Sodium Chloride, and Sodium Phosphate Dibasic Anhydrous, Sterile Water
Pharmacy Notice of Privacy Practices
Q: RiteAway Rx Pharmacy Notice of Privacy Practices
THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS
INFORMATION. PLEASE REVIEW IT CAREFULLY.
As part of the federal Health Insurance Portability and Accountability Act of 1996, known as HIPPA, the facility has created this Notice of Privacy Practices. This notice describes the facility’s privacy practices and the rights you, the individual, have as they relate to the privacy of your Protected Health Information (PHI). Your PHI isv information about you, or that could be used to identify you, as it relates to your past and present physical and mental health care services. The HIPPA regulations require that the facility protect the privacy of your PHI that the facility has received or created. The facility will abide by the terms presented within this Notice. For any uses or disclosures that are not listed (including marketing or selling of PHI), the facility will obtain written authorization from you for the use or disclosure, which you will have the right to revoke, at any time, as explained in more detail below. The facility reserves the right to change the facility’s privacy practices and this Notice. The following is an accounting of the ways the facility is permitted, by law, to use and disclose PHI. Uses and disclosures of PHI for treatment: The facility will use the PHI received to fill your prescription and coordinate or manage your health care. Uses and disclosures of PHI for payment. The facility will disclose your PHI to obtain payment. Uses and disclosures of PHI for health care operations: The facility may use the minimum necessar y amount of your PHI to conduct quality assessments improvement activities and evaluate the facility Workforce. The following is an accounting of additional ways in which the facility is permitted or required to use or disclose your PHI without your written authorization. Uses and disclosures as required by law: Uses and disclosures for public health activities. The facility may use or disclose your PHI to a public health authority that is authorized by law to collect for the purpose of preventing or controlling disease, injury, or disability. This includes the FDA so that it may monitor any adverse effects of foods, drugs, nutritional supplements and other products as required by the law. Uses and disclosures about victims of abuse, neglect or domestic violence: The facility may use or disclose your PHI to a government authority if it is reasonably believed that you are victim of abuse, neglect or domestic violence. Uses and disclosures for health oversight activities: the facility may use or disclose your PHI to health oversight agencies for health oversight activities which may include audits, investigations, inspections, as necessary for licensure, compliance with civil laws, or other activities the health oversight agency is by law authorized to conduct. Disclosures to individuals involved in your care: The facility may disclose your PHI to individuals involved in your care. Disclosures for judicial and administrative proceedings: The facility may disclose your PHI in the course of any judicial or administrative proceedings, provided that the proper documentation is presented to the facility. Disclosures for law enforcement purposes: The facility may disclose your PHI to law enforcement for authorized purposes as required by law or in response to a court order or subpoena. Uses and disclosures about the deceased: The facility may disclose PHI postmortem, or prior to, and in reasonable anticipation of an individual’s death, to corners, medical examiners and funeral directors. Uses and disclosures for cadaveric organ, eye or tissue donation purposes: The facility may use or disclose your PHI for the purposes of procurement banking, or transplantation of cadaveric organs, eyes, or tissues for donation purposes. Uses and disclosures for research purposes: The facility may use and disclose your PHI for research purposes with a valid waiver of authorization approved by an institutional review board or a privacy board. Otherwise, the facility will require a signed authorization by the individual for all other research purposes. Uses and disclosures to avert a serious threat to health or safety: The facility may use or disclose your PHI in good faith and consistency with any applicable law and standards of ethical conduct, to avert a serious threat to health or safety. The facility may use or disclose your PHI for specialized government functions, including military and veterans’ activities, national security and intelligence, protective services, department of state functions, and correctional institutions and law enforcement custodial situations. Disclosures for disaster relief purposes: The facility may disclose your PHI for disaster relief purposes: The facility may disclose your PHI as authorized by law to a public or private entity to assist in disaster relief efforts and for family and personal representative notification. Disclosures to business associates: The facility may disclose your PHI to the facilities business associates for services that they provide to or for the facility to assist in providing quality healthcare. To ensure the privacy of your PHI, the facility requires all business associates to apply appropriate safeguards to any PHI received or created.
